Explore more stories of innovation, clinical care, education and community outreach at The Ohio State University College of Medicine.
VIEW ANNUAL REPORT
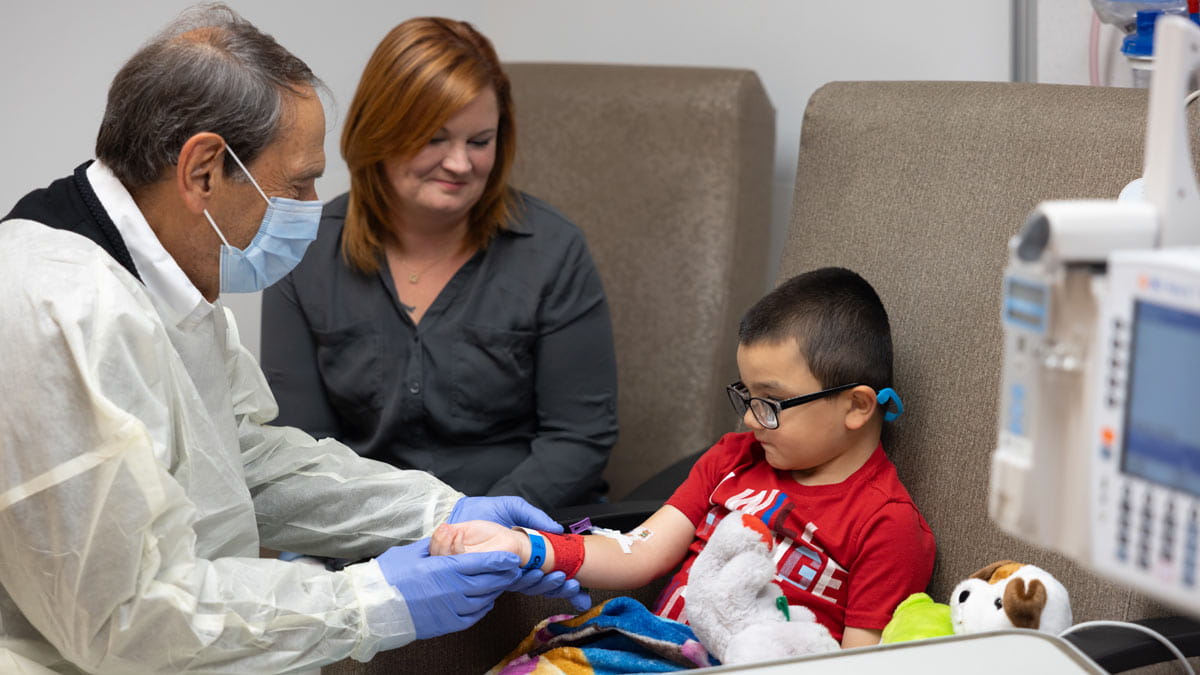 Back in 1969 when Jerry Mendell, MD, was a young neurologist working at the National Institutes of Health, one of his patients was a 7-year-old boy who struggled to get up off the floor.
Back in 1969 when Jerry Mendell, MD, was a young neurologist working at the National Institutes of Health, one of his patients was a 7-year-old boy who struggled to get up off the floor.
Dr. Mendell couldn’t do a lot for him, and that bothered him. At the time, there weren’t any treatments for what the boy had, Duchenne muscular dystrophy, a rare and inherited disease that damages and weakens muscles, including the heart and lungs.
In June, after five decades researching how to stop the disease, Dr. Mendell, a Professor Emeritus in the Department of Pediatrics at The Ohio State University College of Medicine, had a breakthrough. The gene therapy treatment he co-developed with Louise Rodino-Klapac, PhD, received approval from the U.S. Food and Drug Administration for children aged 4 to 5. It’s the first gene therapy treatment the FDA has approved for Duchenne muscular dystrophy.
Balloons and champaign awaited Dr. Mendell outside his office door on June 22, the day the FDA’s decision was made public. He got teary-eyed.
“I had dreamed of this possibility,” he says. “Fifty years of work went into this.”
Duchenne muscular dystrophy is caused by a mutation in a gene that produces dystrophin, a protein that strengthens muscle fibers and protects them from being injured when muscles contract and relax. Without dystrophin, muscle cells die and aren’t replaced. Duchenne primarily afflicts boys, hindering them from walking, running, even standing.
The gene therapy treatment Drs. Mendell and Rodino-Klapac developed is a one-time infusion of Elevidys, artificially made genes that can produce dystrophin and prevent further muscle deterioration.
Since 2004, Dr. Mendell has been principal investigator in the Center for Gene Therapy at the Abigail Wexner Research Institute at Nationwide Children’s Hospital in Columbus. But much of his early career he spent at The Ohio State University College of Medicine. In 1972, he was hired to teach and do research. He carried out the first gene therapy trial in muscular dystrophy on campus in 1999. His early efforts in gene therapy were supported by the Muscular Dystrophy Association (Jerry Lewis' Labor Day Muscular Dystrophy Association Telethon). Funding from many other sources - including philanthropic - later supported the trials leading up to their FDA approval of the gene therapy treatment.
“Every step of the way we learned something,” Dr. Mendell says of his many years and studies testing treatments.
With the FDA approving the gene therapy treatment for 4 to 5 year olds, Dr. Mendell says he continues to work to get the agency’s approval of Elevidys for older and younger children so their lives won’t be limited so severely.
In clinical trials of Elevidys, Drs. Mendell and Rodino-Klapac have treated over 200 patients with Duchenne muscular dystrophy. For the majority of them, the treatment has significantly changed their lives, allowing them to run, stand and walk without struggling.
“I get a hug from these boys, and that’s when the tears come,” he says of his own emotional response to seeing his patients after they’ve been treated. “You know that you’ve changed their lives.”
A longtime partnership between The Ohio State University College of Medicine and Nationwide Children’s Hospital encourages collaboration on research and offers critical training for medical students and resident physicians in the care of pediatric patients.
Many physicians at Nationwide Children’s Hospital, including pediatricians and surgical specialists, also hold faculty appointments at the Ohio State’s College of Medicine.
Nationwide Children’s provides a diversity of educational experiences at the bedside, in the clinic, in the community and in the research laboratory.
“The unique pediatric care that a dedicated pediatric hospital provides enhances the educational opportunities for all of our learners,” says Dan Clinchot, MD, vice dean for Education in the Ohio State College of Medicine.
“The partnership and collaboration between the College of Medicine and Nationwide Children’s Hospital accelerates life-changing research” notes Carol Bradford, MD, MS, FACS, dean of the College of Medicine.
Arick Forrest, MD, MBA, vice dean for Clinical Affairs at the College of Medicine, notes that “the complementary clinical programs benefit patients with the added sub specialization and allows for smooth transition of care as patients age.”
At Nationwide Childrens, one of the nation's top pediatric training institutions in the country, students become immersed in the continually evolving field of medicine and complex pediatric cases. They see firsthand how developing therapeutic interventions and novel discoveries change lives.
The College of Medicine and Nationwide Childrens have partnered for over 100 years and have remained committed to educating the next generation of leaders who will work together to improve the health of all children.
Explore more stories of innovation, clinical care, education and community outreach at The Ohio State University College of Medicine.
VIEW ANNUAL REPORT